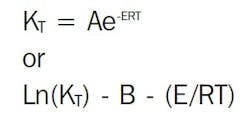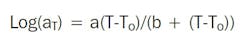When it comes to rubber and polymer hoses, life prediction starts with testing the materials, but demonstration that they are fit for service in specific applications is needed. For polymeric materials, their properties are time- and temperature-dependent and can be significantly affected by the fluids with which they come in contact.
Accelerated laboratory tests can predict service life based on the service conditions a component will experience. These short-term aging tests are suited to rank different polymers in terms of their susceptibility to chemical attack or change in properties; However, unstrained tests do not give any real information related to long-term design data or predictions of expected lifetime when subjected to static or dynamic loads in an extreme environment. The performance of the polymer is often overestimated, resulting in unexpected failure. It is important for engineers to understand the behavior of a polymer in its operating environment to ensure an accelerated testing program is applicable.
Service life
First consider when failure could occur during the product life cycle. It is unknown how long parts will last, but inspection and testing offer valuable insights.
Before testing, consider these difficulties when predicting the service life of a polymer:
- Polymers are time-, temperature-, environment- and stress-dependent.
- The property value(s) at which the polymer fails is (are) often unknown.
- Polymers and service conditions are often variable.
- It is impossible to fully replicate service conditions in accelerated tests.
- Failure mechanisms are not always progressive; A stepwise change in properties can occur.
- Test costs may be prohibitive.
- Long-term testing may be difficult.
New or replacement materials should be benchmarked against materials with known service performance, for more accurante test results. When possible, products should be taken out of service to confirm accelerated testing validity.
Failure modes
Failure can be caused by a range of external factors. Failure modes include:
- Mechanical (creep, fatigue, stress relaxation, impact, environmental stress cracking [ESC] and wear)
- Thermal (degradation, dimensional instability and fire)
- Oxidative (oxygen, ozone and metal catalyzed)
- Chemical (fluids, gases, water and steam)
- Radiation (sunlight, ultraviolet light and atomic radiation)
- Biological (microorganisms)
- Electrical (arcing and electrostatic buildup)
How much these factors affect the polymer depends on many parameters, including polymer type, formulation, test geometry and environmental conditions. A polymer that interacts with the environment can cause a range of mechanisms like cross-linking, chain scission, chemical reactions, change in composition (extraction and absorption) and changes in crystallization. These can be witnessed as cracking, crazing, discoloration, swelling or shrinkage, and as a change in properties.
The challenge is to develop methods that assess the lifetime of the polymer under different service conditions. Generally product tests can better replicate the service conditions.
Accelerated aging
Increasing temperature provides greater energy for reactions to occur in a shorter time frame, but it has limitations, since it can cause different chemical reactions from those at operating temperature. The upper test temperature is set below the maximum operating temperature of the polymer, and can be confirmed by differential scanning calorimetry or dynamic mechanical analysis.
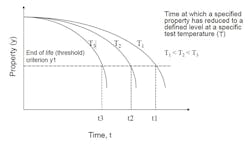
The relationship between the reaction rate of degradation mechanisms and temperature can be determined by exposing test pieces to a series of elevated temperatures. Estimates can then be made by extrapolating the degree of degradation after a given time at a given temperature or the time, at a given temperature, it takes to reach a given degree of degradation.
Two established models describe the relationship between reaction rate and temperature: the Arrhenius equation and the time-temperature superposition. The Arrhenius equation is the best known and most widely used, but it is not suited for all life prediction work. The practical difference between the two methods is that a specified level of reaction rate does not need to be chosen for time-temperature superposition.
Arrhenius model for lifetime prediction
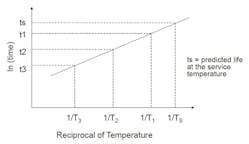
The reaction-rate-temperature relationship can be represented by the Arrhenius equation:
If the model is valid, plotting the natural log of the reaction rate (t1, t2, t3) against the reciprocal of the absolute test temperature (T1, T2, T3) should result in a straight line. This can be extrapolated to determine life to the service temperature (Ts).
Service life is easily changed by choosing a different property, end-point criterion or test-piece geometry. Additional test temperatures and samples can improve the accuracy of the prediction. Extrapolation should also be limited to 30°C to 40°C beyond the last data point since the rate of reaction doubles with every 10°C increase.
Model of time-temperature superposition
An alternative approach to the Arrhenius plot is to shift the plots of property against time along the time axis to construct a master curve. The time shifts can be done by eye or by using mathematical models such as the Williams-Landel-Ferry (WLF) equation:
Variable aT is the shift factor of an isotherm determined at temperature T in relation to the isotherm at the reference temperature To, and a and b are adjustable coefficients, depending on the material. Standard curve-fitting techniques are used to determine the best fit for the WLF equation to give the values of coefficients a and b.
The model can only be used when the curve form is essentially the same at the different temperatures.
Test methods in the stress-free state
Thermal/oxidative
The upper operating temperature or service life of a polymer can be derived from long-term aging tests. Numerous international standards for heat aging exist:
- ISO 188/ASTM D573/ASTM D3045: Heat aging
- BS EN 60216-1 /ISO 2578/UL 746B: Determination of thermal endurance index
- ISO 11346: Estimation of lifetime and maximum service temperature
All involve exposing standard test pieces to heat in an oven with controlled air flow and measuring the change in mechanical or physical properties with time. Controlling the oven controls the oxidation rate, which can influence the results. Chemical analysis can be used to monitor changes in composition or structure.
Once aging tests are performed on materials compounded with antioxidants, a stepwise change in degradation can occur. This can result in additional cross-linking, further complicating test interpretation.
Fluid resistance
Standard test methods for evaluating the resistance of polymers to the action of fluids are detailed in ISO 1817/ASTM D471: Resistance to fluids. Both methods expose test pieces to fluid under defined temperature, time and pressure. Fluid can have physical and chemical effects on a polymer. Full-immersion tests are often more aggressive than service conditions because the polymer may only be partially exposed.
Cross-linked polymers are generally not soluble, but fluid can cause changes in the physical properties through absorption and extraction of soluble constituents. Swelling occurs if absorption is greater than extraction, and shrinkage occurs if the opposite is true.
Without a chemical reaction, the change in volume increases to a point where no more fluid can be absorbed and volumetric expansion remains constant. The time taken to reach equilibrium depends on the diffusion coefficient of the material, temperature, shape and thickness of the part.
Higher test temperatures increase volume change. For example, some oils that cause shrinkage at a lower temperature can cause swelling at higher temperatures. The rate of reaction also increases with increasing temperature. Different reactions can occur at different temperatures and after extended periods, especially if protective agents in the polymer become depleted with time. An indication of a chemical reaction is when the volume change cannot achieve equilibrium with time.
Long-term aging under load
While aging methods determine if a difference occurs after exposure, the viscoelastic nature of polymers means that premature failure can happen without a measurable change. Static or intermittent/cyclic loading will cause changes in the microstructure over time that manifest as creep or fatigue failure.
Chemical environments can penetrate a polymer’s microstructure during tensile stress. Microcrazes form at the surface and can form cracks, which can satisfy brittle failure criteria. For creep and fatigue failures, the time-to-failure is accelerated by elevated temperature, higher stress and more aggressive chemical environments. Chemical compatibility tests may not highlight ESC effects because specimens are usually unstressed during exposure.
Long-term mechanical behavior
Short-term tests can result in long-term performance predictions. Creep strain or time-to-failure data can be measured for static or dynamic loading, with additional tests carried out above the maximum operating temperature. Characteristic curves can then be used to construct a master curve, extending to longer timescales using time-temperature superposition.
Valuable engineering design data from this testing can more accurately predict part performance in service, but it is better suited to the qualification of final material choice. It is possible to use monotonic creep tests to investigate the susceptibility of different materials or chemical environments to environmental stress-cracking or premature rupture.1 During this tensile test, stress is increased at a constant rate while the level of strain is measured. The result is a stress-strain curve measured over a period of 12 to 24 hours. If the chemical environment acts as an ESC agent, a deviation from the air reference is seen.
Concluding remarks
Standard aging and compatibility tests are most suited for materials that will not be subjected to stress during service. If significant differences are shown during aging, the performance of the material must be questioned.
These tests tend not to highlight any ESC effects caused by the combination of stress and the chemical environment. Therefore, to minimize the risk of apparently unforeseen failures and to improve design confidence, it is recommended to always consider long-term mechanical properties in the operating environment and temperatures.
Once materials and the appropriate construction have been selected, testing of the completed hose assembly is critical to ensure it meets specifications and can withstand operating conditions. Certain factors can affect the service life of the final product, such as flexing the hose smaller than its specified minimum bend radius, damage like twisting or kinking, operating above or below a specified temperature range, exposure to impulse pressures greater than the maximum allowable working pressure and intermixing hoses and connectors that are not recommended by the manufacturer.
Author’s note: This feature is based on a paper entitled “Life Prediction of Polymers for the Oil & Gas Industry, which was originally presented in Aberdeen, Scotland, U.K., April 17-18, 2012, at the High Performance Elastomers and Polymers for Oil & Gas Applications conference organized by Smithers Rapra.
Reference
- Hough, M.C. & Wright, D.C. (1996) Two new test methods for assessing the environmental stress cracking of amorphous thermoplastics, Polymer Testing 15, pp. 407-421.
Dr. Andrew Hulme and Jenny Cooper work for Smithers Rapra. Since 2001, Dr. Hulme has been a principal consultant in plastics at Smithers Rapra, providing independent advice on plastics design and manufacturing to end users and designers of plastic components in all industries, including aerospace, oil and gas, and consumer goods. This includes carrying out many successful investigations to determine cause of failure and identify suitable cost-effective solutions. Cooper is the commercial manager for the provision of technical services to support the Industrial Sector at Smithers Rapra. Her clients include oil and gas companies, raw material suppliers, compounders and distributors, utility companies, electrical and electronic device manufacturers and companies in the construction industry. She has published several technical articles and presented conference papers on challenges faced by companies in the sector, including durability and life prediction.